Written by Mugdha Rairikar, Priyadarshini John, Shweta Shah
Clinical Vignette:
A 16 yr old male with schizophrenia, on long-term lithium therapy presented with emesis, progressive generalized edema, altered mental status and oliguria. He denied accidental or intentional medication overdose and admitted to using ibuprofen 600 mg as needed for nonspecific pain. His PE was notable for GCS 11, BP 140/88 mmHg, HR 104 bpm, RR 16/min and SpO2 95% on room air.
He had acute kidney injury (AKI) with creatinine of 2.67 mg/dL (236 umol/L), BUN 64mg/dL (22.8mmol/L), Na 131 mEq/L (131mmol/L), albumin 2.6 g/dL (26g/L) and UPC 7.4mg/mg.Initial lithium level was 3.7 mmol/L (normal therapeutic range 0.8-1.2mmol/L). Over the next two days, his creatinine worsened to 4.2mg/dL(371umol/L). He developed oliguria, worsening fluid overload and persistent hypertension; his lithium level persisted above 2.5 mmol/L. His psychotic symptoms continued along with waxing and waning sensorium. He was therefore started on continuous kidney replacement therapy for AKI and lithium toxicity.
The vignette highlights several key points:
1. GI symptoms heralding acute toxidrome
2. AKI likely multifactorial from decreased kidney perfusion due to dehydration NSAID exposure and lithium toxicity
3. AKI causing decreased clearance and persistent supratherapeutic levels
4. Lithium toxicity can present with nephrotic syndrome
5. Need for kidney replacement therapy due to worsening symptomatic AKI and symptomatic high lithium levels
For decades, lithium has been the most potent medication for clinical improvement and suicide prevention in psychiatric disorders like bipolar disorder, schizophrenia.However, its use is largely limited by its narrow therapeutic index and long-term effects on kidneys that might persist despite discontinuation of therapy. The recommended target serum level of lithium for acute and maintenance therapy is 0.8 to 1.2 mmol/L. Ideally it is recommended that trough serum lithium levels are checked 5 to 7 days after each dose escalation.
Acute lithium toxicity often presents with gastrointestinal symptoms and neurologic symptoms develop later. Acute Kidney Injury is not uncommon in the presence of other risk factors such as dehydration, NSAID use and CKD. Chronic lithium toxicity predominantly presents with renal involvement in the form of nephrogenic diabetes insipidus in >80% of patients and with chronic neurologic involvement.
Figure 1 : History of lithium discovery and usage in medical practice

Mechanism of action of Lithium:
Blocks two important signaling pathways- inositol monophosphate and glycogen synthase kinase-3 (GSK3).
Modulates neurotransmission by inhibiting excitatory neurotransmitters like dopamine and glutamate and stimulating GABA mediated neurotransmission.
Enhances the production of neuroprotective factors like BDNF and Bcl2 by enhancing cAMP response element binding protein-transcription factor( CREB-TF).
Risk factors for lithium induced nephrotoxicity:
Age-Elderly individuals
Hypovolemia
Prolonged lithium exposure
Low baseline kidney function
Presence of other comorbidities like HTN and DM
Concomitant use of other nephrotoxic drugs, NSAIDS, diuretics etc
Past history of lithium toxicity
Effects of lithium on Kidney:
The prevalence of lithium nephrotoxicity was observed to be 1.2% in a Swedish study. Duration of therapy and cumulative dose of lithium were some of the risk factors implicated in this particular study. Lithium can affect kidneys in several ways. Most common is nephrogenic diabetes insipidus. It is infrequently associated with glomerular diseases like minimal change disease, focal segmental glomerulosclerosis, tubular disorders like renal tubular acidosis, chronic tubulointerstitial nephropathy and electrolyte disorders like hypercalcemia.
Lithium induced podocyte injury is a rare and less understood complication of chronic lithium use. Lithium causes nephrotic syndrome by disruption of glomerular basement membrane barrier and foot processes of the podocytes by-
T-lymphocyte activation and secretion of tumor necrosis factor
Activation of phosphoinositol pathway resulting in T cell stimulation.
Markowitz et al. studied 24 cases of biopsy proven lithium nephrotoxicity, all of which had some form of kidney dysfunction. Of those, 41.7% had proteinuria and 87% had Nephrogenic Diabetes Insipidus (NDI). Renal histology showed cysts arising from distal tubule and collecting ducts with features of chronic tubulointerstitial nephritis (CTIN). 50% of the patients had secondary FSGS which is probably due to nephron loss and compensatory hypertrophy of the remnant nephrons, along with features of CTIN. Most often proteinuria resolves upon discontinuation of lithium.
Nephrogenic Diabetes Insipidus (NDI): Downregulation of apical aquaporin-2 (AQP2) channels in collecting tubules by the following proposed mechanisms.
Epithelial Sodium channel (ENaC) mediated entry of lithium into the cell across the membrane leads to the following
Decreased ADH receptor sensitivity (20-40%)
Decreased expression of AQP2
The mechanisms by which reduced ADH receptor sensitivity and AQP2 expression occurs is outlined below;
GSK3 mediated inhibition of of cellular processes activating AQP2 expression via serine phosphorylation
Increased cyclo-oxygenase-2 (COX-2) resulting in increased prostaglandin E2 in urine through the below mechanisms;
Decreased AQP2 gene expression
Principal cell cycle arrest → apoptosis
Clinically, patients present with polyuria, polydipsia and nocturia. However, central DI associated with psychotropic medications and primary polydipsia should be considered in the differential diagnosis. NDI is partially reversible initially, but with prolonged use of typically over 2 decades could be irreversible.
Treatment:
Discontinuation of therapy or reduction in dose, if possible.
Diuretics
Amiloride,potassium sparing diuretic (mild-moderate concentrating defect)
Acts by inhibiting ENaC channel function, thereby limiting lithium influx into principal cells.
Thiazide diuretics, although exact mechanism is unclear, several mechanisms are proposed that work in combination with a low salt diet:
Increases sodium loss by blocking the sodium chloride (NaCl) transporter in distal convoluted tubule resulting in increased proximal tubular reabsorption of sodium and water.
Upregulation of AQP2 receptors in the collecting tubule.
If there is diuretic mediated volume depletion leading to increased proximal sodium mediated lithium absorption, lithium dose reduction may be helpful.
Other proposed therapies include
Nonsteroidal anti-inflammatory drugs (NSAIDs) - act by inhibiting COX enzyme and decreasing the synthesis of prostaglandins.
Vasopressin to overcome partial resistance.
3. Renal cysts are formed due to enhanced uptake of lithium by principal cells. Tubular dilation, mainly that of distal tubules and collecting ducts, leads to the cyst formation. Cysts are present amid the background of CTIN and glomerulosclerosis and are generally 1-2 mm in diameter, distributed in both the cortex and medulla. They appear echogenic on ultrasound, non-enhancing on CT, and hyperintense on T2 MRI.
4. Lithium is known to cause a low anion gap acidosis and elevated osmolar gap. The mechanism of lithium induced distal renal tubular acidosis is unclear but is presumably caused by increased secretion of ammonia.
5. PTH-dependent and independent hypercalcemia. Lithium possibly causes parathyroid gland hyperplasia through GSK3 inhibition and irregular Wnt/β-catenin signaling. The PTH-independent mechanism is mediated via down regulation of calcium sensing receptors.
6. Kidney failure secondary to lithium is uncommon and usually occurs after prolonged use >20 years. ANZDATA from the year 2000 observed that 0.2-0.7% of kidney failure that year was ascribed to lithium. Unfortunately, this is progressive even after cessation of lithium. The observed decline of eGFR of ~0.92 mL/min/1.73 m2 per year in individuals treated with lithium. In this study, the predominant histopathology in lithium users was CTIN.
Figure 2 shows the lithium handling by the kidneys and a summary of lithium induced renal complications

Prevention of lithium toxicity:
Once a day dosing of non-long acting preparation, unlike the sustained release of lithium.
Nocturnal administration of the drug has the added advantage of achieving the target level with 20% reduction in the daily dose requirement as the kidneys tend to filter lithium slowly in the night.
Guidelines for monitoring Lithium toxicity:
The UK National Institute for Health and Care Excellence (NICE) and the American Psychiatric Association (APA), recommend 3- 6 monthly monitoring of eGFR, proteinuria during the initiation phase of lithium followed by annual monitoring in patients on maintenance therapy. More frequent monitoring is recommended in elderly patients and in those who are receiving concomitant nephrotoxic drugs. Nephrology consult is sought once CKD stage 3 sets in or a progressive decline in eGFR >4ml/min/m2.
Treatment of Lithium Toxicity:
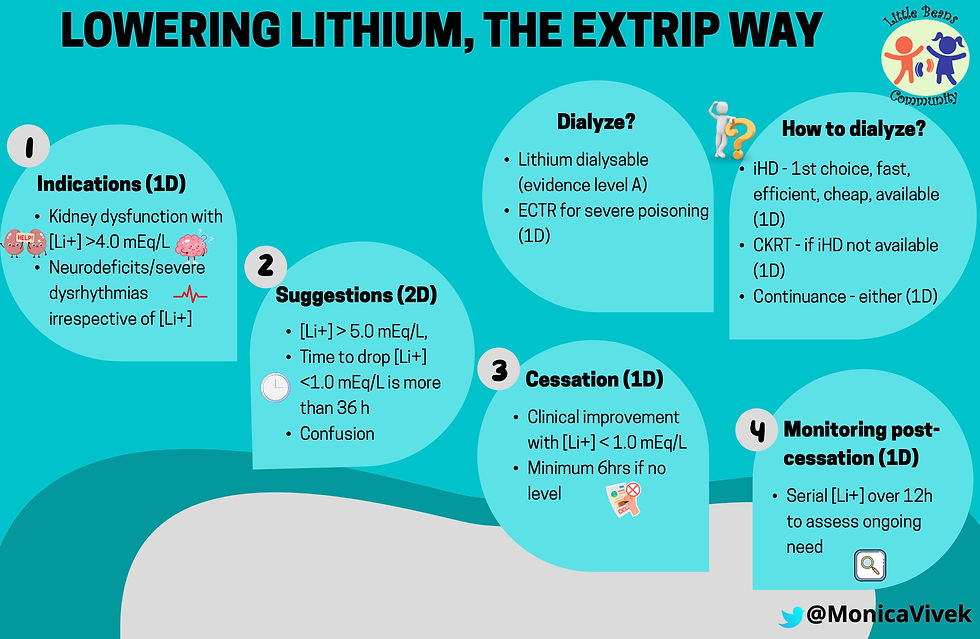
Restoring intravascular volume with normal saline in patients with dehydration and ongoing gastrointestinal losses is important to prevent acute kidney injury and/or worsening. Monitoring serum sodium levels for hypernatremia especially in patients with NDI to determine free water replacement in addition to maintenance fluids is equally vital. Extracorporeal therapy is indicated per EXTRIP recommendations as below.
EXTRIP Recommendations:
The Extracorporeal Treatment (ECTR) in Poisoning Workshop provides evidence based guidelines on the use of extracorporeal therapies in cases of poisonings as below.
Take Home Message:
Lithium has long been used for treatment of major psychiatric disorders but has a narrow therapeutic window and significant acute and long term nephrotoxicity necessitating regular monitoring of levels while on therapy.
Nephrogenic Diabetic Insipidus is most common renal manifestation.Progressive tubulointerstitial nephritis and ESKD can rarely develop due to chronic use of lithium
Lithium is dialyzable and hemodialysis is recommended for acute symptomatic lithium toxicity.

.png)
Kommentare